Streamlined Delivery System
The Somatuline Depot delivery system was redesigned in 2019 to help streamline administration.1,12
- Prefilled deep subcutaneous injection needs no reconstitution1
- Somatuline Depot should be administered by a healthcare professional1
- Allow 30 minutes for product to come to room temperature1
- Product may be returned to the refrigerator for continued storage and used at a later time if left in its sealed pouch at room temperature (not to exceed 104℉ or 40℃) for up to 24 hours1
- Automatic needle guard is designed to retract the needle once the plunger is depressed and thumb is removed1
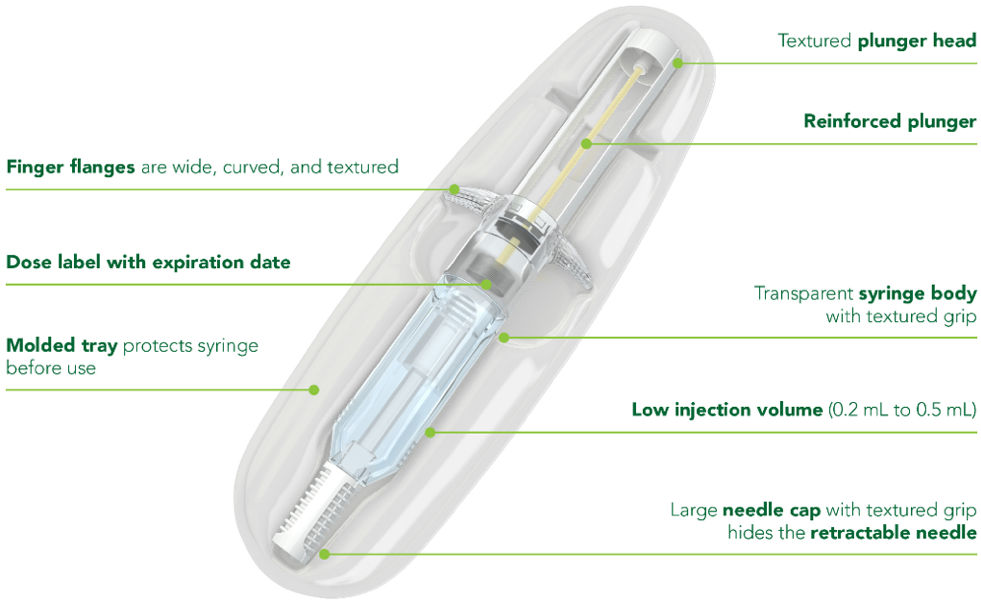
Device not shown actual size
The Somatuline Depot delivery system was updated in 2019 to provide an improved ergonomic injection experience, based on user feedback. Through a series of 4 formative studies between 2015 and 2017, Ipsen sought feedback from acromegaly and NET patients, nurses and caregivers on the design and functionality of new delivery device prototypes. These culminated in a human factors validation study in 2017 in which the final delivery system prototype was tested to determine whether the product could be safely and effectively used by intended users in the intended use environment. Key changes between the prior delivery system and the current delivery system (updated in 2019) are: an overcap to improve the ergonomics (and needle shield removal); plunger support for the new delivery system; and improved version of the needle safety system.
How to Administer Somatuline Depot Using the 2019 Redesigned Syringe
Dosing Somatuline Depot

*Controlled is defined as GH level from >1.0 ng/mL to ≤2.5 ng/mL, normalized IGF-1 level, and satisfactory management of clinical symptoms as determined by the healthcare professional.