- ADMINISTRATION
- Administration
HOW TO ADMINISTER SOMATULINE DEPOT (LANREOTIDE)
How to use the syringe
Somatuline Depot (lanreotide) is intended to be administered by a healthcare provider through deep subcutaneous injection only. Learn how to use Somatuline Depot syringe by downloading the administration guide below.

DO remove from refrigerator and let sealed pouch sit for 30 minutes to reach room temperature*
DON’T open sealed pouch or inject medication that has not yet reached room temperature; injecting cold medication may be painful for your patient
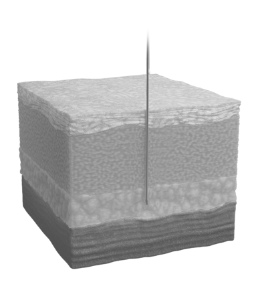
DO administer deep subcutaneous injection only
DON’T administer medication intramuscularly. If you do, do not administer another dose
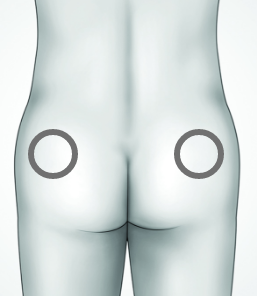
DO administer in the upper outer quadrant of the right or left buttock
DON’T administer in the arm, calf, abdomen, or any other part of the body
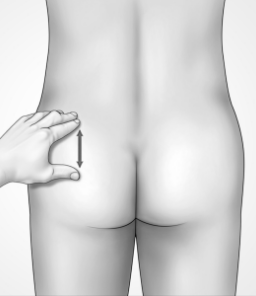
DO alternate injection sites between appointments
DON’T inject in the same location as the last appointment
DO flatten the area by using your thumb and index finger to spread the skin
DON’T fold, squeeze, or pinch the skin
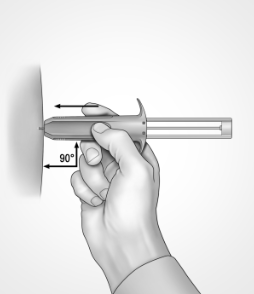
DO insert needle at a 90 degree angle only, and all the way into the skin (the needle should not be visible)
DON’T aspirate (draw back the plunger) before injecting, or inject at an angle other than 90 degrees
*Product left in its sealed pouch at room temperature (not to exceed 104°F or 40°C) for up to 24 hours may be returned to the refrigerator for continued storage and use at a later time.1
REFERENCE:
- Somatuline Depot (lanreotide) Injection [Prescribing Information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc.; July 2024.

